Morpholino Types for Therapeutic Oligonucleotides
Morpholinos are synthetic molecules utilized in antisense morpholino-based oligonucleotides designed to bind to specific RNA sequences to block biological processes, such as mRNA translation into protein. Unlike regular DNA or RNA oligonucleotides, morpholinos have a modified backbone of morpholine rings linked by non-ionic phosphorodiamidate bonds. This modification makes PMOs highly stable and resistant to degradation by cellular enzymes, such as nucleases. Morpholinos have been used in model organisms such as zebrafish, Xenopus (frogs), and mice for research purposes.
In the context of oligonucleotide chemistry, morpholinos and thiomorpholinos contain backbone modification allowing the design of chimeras to modify the stability, binding affinity, and cellular uptake properties of therapeutic oligonucleotides.
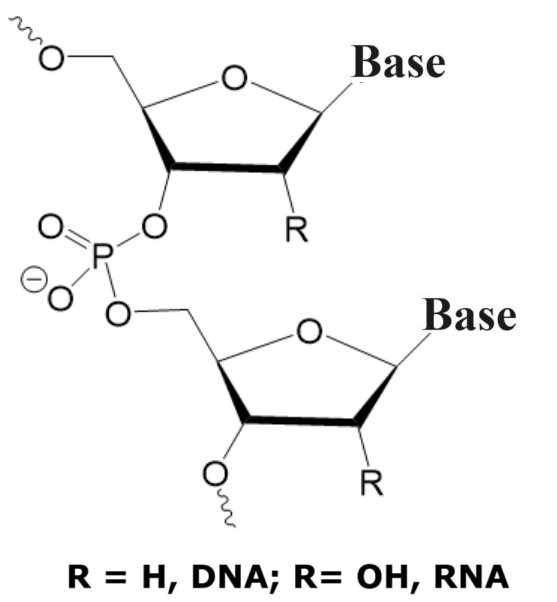
Building Block Structures of DNA/RNA, Phosphorodiamidate Morpholino, and Thiomorpholino Oligonucleotides.
| 
| 
Phosphorodiamidate Morpholino (PMO) | 
Thiophosphoroamidate Morpholino (TMO) | 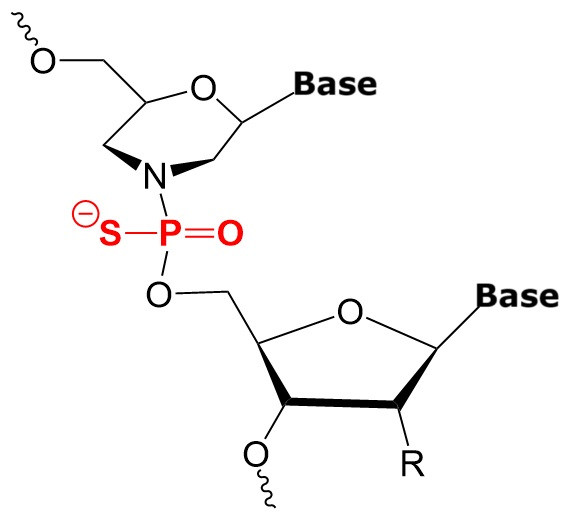
TMO-DNA/RNA Chimeras |
.
Features of morpholinos
Mechanism of action: PMOs can block access to RNA molecules including mRNA by binding to specific sequences, inhibiting translation, or modifying splicing.
Advantages: PMOs are stable, do not elicit immune responses, and are highly specific, reducing off-target effects.
Applications: Morpholinos are widely used in developmental biology, gene knockdown experiments, and therapeutic approaches to silence disease-causing genes.
Limitations: However, the delivery into cells can be challenging. Hence, unique delivery methods like electroporation, microinjection, or specific carrier molecules are often required.
Types of Morpholinos
There are several types of morpholinos, each designed for specific applications.
Phosphorodiamidate morpholinos (PMOs)
Phosphorodiamidate morpholino oligomers (PMOs) are synthetic analogs of DNA or RNA, designed for use in antisense therapy. Unlike natural nucleic acids, PMOs have a modified backbone where the sugar-phosphate structure is replaced by a phosphoramidate linkage and the ribose is replaced by a morpholine ring, enhancing the molecule's stability and resistance to nucleases, making PMOs highly stable in biological environments. Phosphorodiamidate morpholinos are commonly synthesized using a linear synthetic method.
Thiomorpholinos (TMOs)
Thiomorpholinos (TMOs) are sulfur-containing analogs of morpholine, where a ribose is rplaced by a morpholine ring and one of the non-bridging oxygen atoms on the phosphor atom is replaced by sulfur. This modification often introduces differences in chemical properties, such as increased lipophilicity and different reactivity patterns, which can be useful in drug design, especially in improving membrane permeability and modifying metabolic stability.
TMOs are morpholino nucleotides with a thiophosphoramidate inter-nucleotide linkage instead of phosphorodiamidate in PMO. Standard solid phase synthesis using phosphoramidite chemistry allows the synthesis of TMOs from the 3' - to the 5’-end.
Due to this greater flexibility in synthesis, TMOs can target a broader range of RNAs, compared to PMOs, by incorporating different RNA/DNA modifications. Also, TMOs have enhanced chemical stability, making them more resistant to nucleases. The chemical stability prolongs their activity in biological systems. Compared to PMOs, the thiol modifications in TMOs can lead to stronger binding to target RNA, potentially increasing their effectiveness in gene regulations. The design of TMOs allows for more specific targeting, minimizing off-target effects and unintended interactions with non-target sequences.
TMOs allow for regulating exon skipping activities, for example, in Marfan Syndrome and Duchenne Muscular Dystrophy, and in regulating TUG 1 RNA.
TMOs perform well at lower concentrations for exon 23 skipping and exhibit excellent potency compared to PMOs and other modifications.
Compared to MOE modifications, a TMO gapmer has a higher allele selective knockdown of the "fused in sarcoma" (FUS) gene.
Standard Morpholinos
Standard morpholinos are unmodified morpholinos used primarily for research purposes. They bind to complementary RNA sequences, blocking translation or splicing by steric hindrance.
Standard morpholinos are often used in:
Gene knockdown studies: Preventing the production of specific proteins by inhibiting mRNA translation.
Splice-modifying studies: Blocking the splicing of pre-mRNA to study the role of certain splice variants.
Cell-penetrating peptide–morpholinos PPMOs
Cell-penetrating peptide–morpholinos are a type of morpholino oligonucleotide that are covalently conjugated with an arginine-rich peptide also known as vivo-morpholino.
Vivo-Morpholinos
Vivo-morpholinos are designed for delivery into tissues or whole organisms. Vivo-morpholino oligonucleotide contain an octa-guanidine dendrimer attached via a triazine core. Vivo-morpholinos can be peptide conjugates to enhance cell permeability and uptake.
Vivo-morpholinos are used in:
In vivo gene knockdown: Used in animal models, these morpholino conjugates provide better uptake than standard morpholinos.
Therapeutic applications: Vivo-morpholinos potentially allow the treatment of diseases like Duchenne Muscular Dystrophy (DMD) by altering splicing or blocking harmful RNA transcripts.
Photo-morpholinos
Photo-morpholinos contain a photo-cleavable group that inactivates the molecules until exposed to light, allowing spatial and temporal control over gene knockdown or RNA blocking. Photo-morpholinos are used in:
Precise gene knockdown: Light activation allows researchers to control when and where gene silencing occurs in tissues or embryos.
Developmental biology studies: Photo-morpholinos offer precise control during critical stages of embryonic development.
Lipid-conjugated Morpholinos
Lipid-conjugated morpholinos are coupled with lipophilic groups (e.g., cholesterol) to improve cellular uptake and stability. These are ideal for:
Systemic delivery: Lipid-conjugated morpholinos are used for in vivo applications where efficient tissue penetration is needed.
Increased stability: Lipid-conjugated morpholinos are tend to be more stable in biological fluids, enhancing their effectiveness in live organisms.
End-modified Morpholinos
End-modified Morpholinos have chemical modifications at their 3’- or 5’-ends, improving stability, binding affinity, or cellular uptake. End-modified Morpholinos are used in cases where standard morpholinos may degrade or have low efficacy.
Antisense Morpholinos
Antisense morpholinos target and block complementary RNA sequences, preventing the translation of specific genes. These are mainly used in research to:
Study gene function.
Validate potential therapeutic targets.
The type of morpholino is selected based on the selected application, whether it be for research, in vivo studies, or potential therapeutic development.
To investigate more, follow these links:
Custom-Morpholinos
Thiomorpholino-Oligonucleotides-or-TMOs
Thiomorpholino Oligonucleotides
Reducing-off-target-effects-of-the-sirna-therapeutics
A-Collection-of-Approved-Antisense-Therapeutic-Drugs-2024
---...---
Bio-Synthesis provides custom morpholinos including Phosphorodiamidate Morpholino, and Thiomorpholino Oligonucleotides.
Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and inter-nucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---