Lipid nanoparticles (LNPs) are tiny, spherical molecular carriers ranging in diameter from 1 to 100 nanometers and are widely used for drug and gene delivery, including RNA, DNA, and drugs, but recently, particularly for mRNA vaccines and therapeutics. Compared to traditional delivery methods, because of their improved stability and cellular uptake of nucleic acids, LNPs are now preferred for vaccines.
Their formulation typically consists of four main lipid type:
Ionizable Lipids: Ionizable lipids are essential for encapsulating nucleic acids such as mRNA and siRNA. Ionizable lipids facilitate endosomal escape. Ionizable lipids remain neutral at physiological pH but become positively charged in acidic environments, aiding cellular uptake. Examples are DLin-MC3-DMA (used in Onpattro®), ALC-0315 (used in Pfizer-BioNTech COVID-19 vaccine), and SM-102 (used in Moderna COVID-19 vaccine).
Phospholipids: Phospholipids provide structural stability and aid in forming the bilayer or vesicle. Facilitate membrane fusion and lipid organization. Examples are DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine) and DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine).
Cholesterol: Cholesterol enhances the stability and rigidity of LNPs. Reduces leakage of encapsulated cargo and can be modified for targeted delivery (e.g., cholesterol derivatives).
PEGylated Lipids (Polyethylene Glycol-Lipids): PEGylated lipids improve circulation time by reducing immune clearance and control particle size and prevent aggregation. Examples are PEG2000-DMG (1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000) and PEG2000-DSPE (1,2-distearoyl-sn-glycero-3-phospho-ethanol-amine-N-[methoxy(polyethylene glycol)-2000]).
As an example, for the formulation of vaccine LNPs, the Pfizer-BioNTech COVID-19 vaccine is formulated using the functional lipids ALC-0315 and ALC-0159, and the structural lipids DSPC and cholesterol together with sucrose and buffers (Source: Pfizer/BioNTech COVID-19 mRNA vaccine (BNT162, PF-07302048) TGA Pre-Submission Meeting September 18, 2020).
Table 1: Lipids for LNPs
| Name | Structure | Characteristics | Function | Ref. |
| Cholesterol | 
| Waxy, fat-like substance essential for the human body. Produced by the liver and cells. | Improves stability and fluidity. | Kawaguchi et al. 2023. |
| DOTMA [N-[1-(2,3-dioleyl-oxy) propyl]-N,N,N-trimethyl-ammonium chloride] | 
| Quaternary ammonium head group. Glycerol-based back bone. Two ether linkage bonds. Two hydrocarbon chains. | DOTMA interacts directly with plasmid DNA, forming lipid–DNA complexes that exhibit 100% entrapment. | Felgner et al. 1987. |
| DOSPA [2,3-dioleyloxy-N-[2-(spermine-carboxamido) ethyl]-N,N-dimethyl-1-propanaminium trifluoroacetate] | 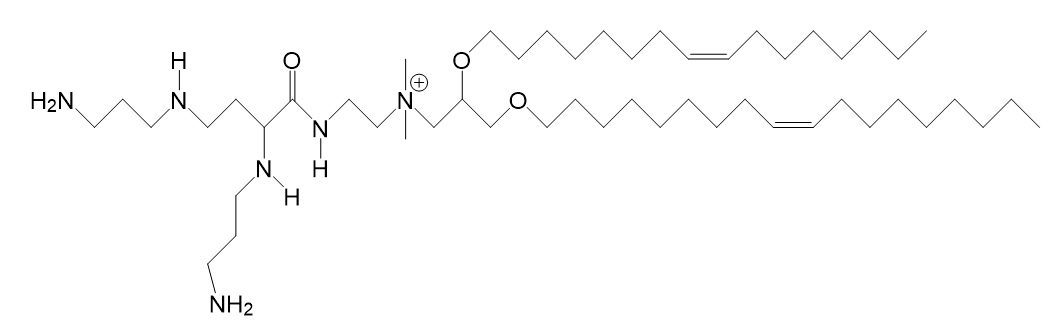
| Quaternary ammonium. Spermine head group bound via a peptide bond to the hydrophobic chains. Two 18-carbon alkyl chains. | Multivalent lipid, containing quaternary ammonium and spermine optimized to deliver mRNA in alveolar cells, cardiac muscle cells and pluripotent stem cells. | Hou et al. 2021. |
| DOTAP [1,2-dioleoyl-3-trimethylammonium-propane] | 
| Quaternary ammonium head group. Glycerol-based back bone. Two ester linkage bonds. Two hydrocarbon chains. | Incorporates degradable ester bonds connecting the cationic head group and hydrophobic lipids exhibited elevated transfection efficiency and reduced toxicity. | Leventis & Silvius 1990. |
| DOPE [1,2-dioleoyl-sn-glycero-3-phosphoethanol-amine] | 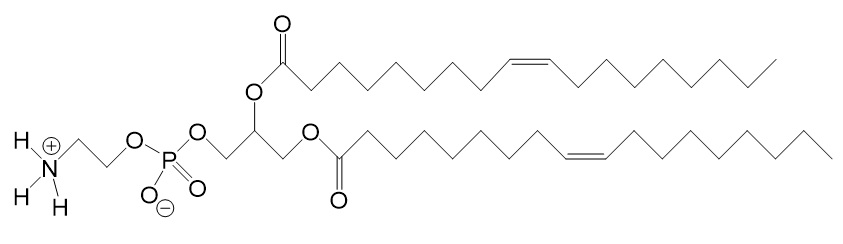
| Primary amine phosphatidylethanolamine head group Double-unsaturated lipid tail. | Improves the transfection efficiency in cationic lipid formulations by promoting membrane fusion. | Evers et al. 2018. |
| DSPC [1,2-distearoyl-sn-glycero-3-phosphocholine] | 
| Quaternized amine phosphatidylcholine head group. Saturated acyl chains. | Phosphatidylcholine with cylindrical geometry that allows DSPC molecules to form a lamellar phase, which stabilizes the structure of lipid nanoparticles. | Veiga et al. 2020. |
| ePC [Ethyl phosphatidyl-choline] | 
| Quaternary nitrogen head group. | Synthesized by introducing a third alkyloxy group into phosphatidylcholines to eliminate negative charge and applied for mRNA-based cancer immune-therapy and protein replacement therapies. | Patel et al. 2019; Zhang et al. 2019. |
| DLin-MC3-DMA [(6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl 4(dimethyl amino) butanoate] | 
| Tertiary amine head group which shows pH-dependent ionization Ester linkers Two linoleyl tails pKa: 6.44 | Employed in the liver’s mRNA delivery process through albumin receptor-mediated mechanisms. | Miao et al. 2020. |
| OF-Deg-Lin [(((3,6-dioxo-piperazine-2,5-diyl) bis (butane-4,1-diyl)) bis(azanetriyl)) tetrakis (ethane-2,1-diyl) (9Z,9′Z,9″Z,9‴Z,12Z,12′Z,12″Z,12‴Z)-tetrakis (octadeca-9,12-dienoate)] | 
| Diketopiperazine core. Alkenyl amino alcohols and containing four biodegradable ester linkages. Doubly unsaturated tails. pKa: 5.7 | Achieving specific and targeted delivery of mRNA into B lymphocytes. | Fenton et al. 2017. |
| OF-C4-Deg-Lin [(((3,6-dioxo-piperazine-2,5-diyl) bis(butane-4,1-diyl)) bis (azanetriyl)) tetra-kis(butane-4,1-diyl) (9Z,9′Z, 9″Z,9‴Z, 12Z,12′Z,12″Z,12‴Z)-tetrakis (octa-deca-9,12-dienoate)] | 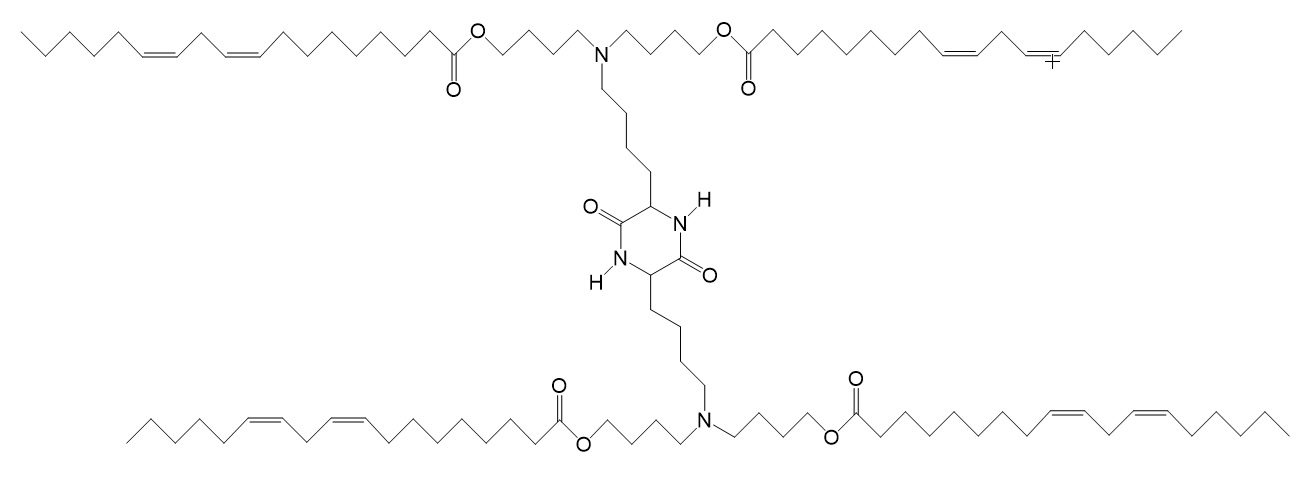
| Diketopiperazine core containing doubly unsaturated tail. Linkers containing a length of four-carbon aliphatic chain. | Precision delivery of siRNAs and mRNAs. | Fenton et al. 2018. |
| ALC-0315 [((4-hydroxy butyl)-azane-diyl) bis(hexane-6,1-diyl) bis(2-hexyldecanoate)] | 
| Tertiary amine head group. Terminal hydroxyl group. Ester linkers. pKa: 6.09 | Ionizable delivery components in the mRNA-1273 and BNT162b COVID-19 vaccines. Nucleic acid complexation and Membrane fusion. | Boldyrev et al. 2023. |
| cKK-E12 [3,6-bis(4-(bis(2-hydroxydodecyl) amino) butyl) piperazine-2,5-dione] | 
| Diketopiperazine core-based head. Four lipid tails. | Multi tail ionizable lipid with enhanced endosome disrupting ability and effectively identified to target hepatic genes. | Han et al. 2021. |
| Lipid H (SM-102) [Heptadecan-9-yl 8-((2-hydroxy-ethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate] | 
| Tertiary amine head group. Terminal hydroxyl group Ester linkers. Two branched saturated tails. pKa: 6.75 | SM-102 contains a primary degradable ester tail and a degradable branched ester tail positively charged at low pH. | Hassett et al. 2019. |
| A2-Iso5-2DC18 [Ethyl 5,5-di((Z)-heptadec-8-en-1-yl)-1-(3-(pyrrolidin-1-yl) propyl)-2,5-dihydro-1H-imidazole-2-carboxylate] | 
| Cyclic amine head group. Dihydroimidazole linker. Unsaturated lipid tail. | Efficiently deliver mRNA and yield effective mRNA expression. | Zhang et al. 2021. |
| 306Oi10 [Tetrakis (8-methylnonyl) 3,3′,3″,3‴-(((methyl-azane-diyl) bis (propane-3,1 diyl)) bis (azane-triyl)) tetra-pro-pionate] | 
| Branched tail. Ionizable lipid tail isodecyl acrylate. | Efficiently co-delivered multiple RNA constructs achieving a transfection rate of over 80% in hepato-cytes, Kupffer cells, and endothelial cells. | Han et al. 2021. |
| BAMEA-O16B [Bis (2-(dodecyl disulfanyl) ethyl) 3,3′-((3-methyl-9-oxo-10-oxa-13,14-dithia-3,6-diazahexacosyl) azanediyl) dipropionate] | 
| Biodegradable lipid integrated with disulfide bonds containing hydrophobic tails. | BAMEAO16B efficiently delivers Cas9 mRNA and sgRNA into cells while releasing RNA in response to the reductive intracellular environment for genome editing. | Liu et al. 2019. |
| PEG2000-DMG [1,2-dimyristoyl-rac-glycero-3 methoxy polyethylene glycol-2000] | 
| PEGylation of myrist-oyl diglyceride. | PEG modifications increase the nanoparticles’ blood circulation duration by diminishing clearance through the kidneys and the mononuclear phagocyte system. | Han et al. 2012. |
| ALC-0159 [2-[(polyethyl-eneglycol)-2000]-N, N ditetradecyl acetamide] | 
| PEGylated lipid consisting of PEG group conjugated to a lipid anchor with two 14-carbon saturated alkyl chains. |
| TCL053 [2-(((4 (dimethyl-amino) butan-oyl)oxy)methyl)-2-((((Z)-tetra-dec-9-enoyl) oxy)methyl) propane-1,3-diyl (9Z,9′Z)-bis(tetradic -9-enoate)] | 
| Three-tailed ionizable lipid. pKa: 6.8 | TCL053 iLNPs transiently deliver CRISPR-Cas9 mRNA and sgRNA to multiple muscle tissues, reducing immune-genicity and increasing the safety of iLNPs. | Kenjo et al. 2012. |
| 246C10 [1,1′,1′′,1′′′-[1,4-piperazinediylbis(3,1-propane-diylnitrilo)] tetra-kis-2-do-decanol] | 
| Lipid based on piperidine. pKa: 6.75 | Buffer preparation of 246C10 iLNPs could increase the encapsulation efficiency of CRISPR-Cas9 mRNA and sgRNA. These iLNPs were able to treat hemophilia safely, without causing hepato-toxicity. | Kim et al. 2021. |
| DOG-IM4 [N-[(25Z)-14-[(9Z)-9-octadecen-1-yloxy]-3,6,9,12,16-pentaoxatetratriacont-25-en-1-yl]-1H-imidazole -5-carboxamide] | 
| Imidazole head and dioleoyl lipid tail. Short flexible polyoxyethylene linker. pKa: 5.6 | Ionizable cationic lipid for the formulation of LNPs displaying increased stability at 4 °C in liquid form and promoting robust mRNA expression and strong immune responses. | Ripol et al. 2022. |
References
Boldyrev I.A., Shendrikov V.P., Vostrova A.G., Vodovozova E.L. A Route to Synthesize Ionizable Lipid ALC-0315, a Key Component of the mRNA Vaccine Lipid Matrix. Russ. J. Bioorg. Chem. 2023;49:412–415. [PMC] [PubMed]
Evers M.J.W., Kulkarni J.A., van der Meel R., Cullis P.R., Vader P., Schiffelers R.M. State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery. Small Methods. 2018;2:1700375. [onlinelibrary]
Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [ PMC]
Fenton OS, Kauffman KJ, Kaczmarek JC, McClellan RL, Jhunjhunwala S, Tibbitt MW, Zeng MD, Appel EA, Dorkin JR, Mir FF, Yang JH, Oberli MA, Heartlein MW, DeRosa F, Langer R, Anderson DG. Synthesis and Biological Evaluation of Ionizable Lipid Materials for the In Vivo Delivery of Messenger RNA to B Lymphocytes. Adv Mater. 2017 Sep;29(33). [PubMed]
Fenton O.S., Kauffman K.J., McClellan R.L., Kaczmarek J.C., Zeng M.D., Andresen J.L., Rhym L.H., Heartlein M.W., DeRosa F., Anderson D.G. Customizable Lipid Nanoparticle Materials for the Delivery of siRNAs and mRNAs. Angew. Chem. Int. Ed. 2018;57:13582–13586. doi: 10.1002/anie.201809056. [PMC] [PubMed]
Han X., Zhang H., Butowska K., Swingle K.L., Alameh M.G., Weissman D., Mitchell M.J. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 2021;12:7233. [PMC] [PubMed]
Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T., et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [PMC] [PubMed]
Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. [PMC] [PubMed]
John R, Monpara J, Swaminathan S, Kalhapure R. Chemistry and Art of Developing Lipid Nanoparticles for Biologics Delivery: Focus on Development and Scale-Up. Pharmaceutics. 2024 Jan 19;16(1):131. [PMC]
Kawaguchi M, Noda M, Ono A, Kamiya M, Matsumoto M, Tsurumaru M, Mizukami S, Mukai H, Kawakami S. Effect of Cholesterol Content of Lipid Composition in mRNA-LNPs on the Protein Expression in the Injected Site and Liver After Local Administration in Mice. J Pharm Sci. 2023 May;112(5):1401-1410. [PMC]
Kenjo E., Hozumi H., Makita Y., Iwabuchi K.A., Fujimoto N., Matsumoto S., Kimura M., Amano Y., Ifuku M., Naoe Y., et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun. 2021;12:7101. [PMC] [PubMed]
Kim M., Jeong M., Hur S., Cho Y., Park J., Jung H., Seo Y., Woo H.A., Nam K.T., Lee K., et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021;7:eabf4398. [PMC] [PubMed]
Leventis R., Silvius J.R. Interactions of mammalian cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim. Biophys. Acta. 1990;1023:124–132. [PubMed]
Liu J., Chang J., Jiang Y., Meng X., Sun T., Mao L., Xu Q., Wang M. Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles. Adv. Mater. 2019;31:e1902575. [PMC] [PubMed]
Miao L., Lin J., Huang Y., Li L., Delcassian D., Ge Y., Shi Y., Anderson D.G. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 2020;11:2424. [PMC] [PubMed]
Nanoparticle
Patel S., Ryals R.C., Weller K.K., Pennesi M.E., Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release. 2019;303:91–100. [PMC] [PubMed]
Ripoll M., Bernard M.C., Vaure C., Bazin E., Commandeur S., Perkov V., Lemdani K., Nicolaï M.C., Bonifassi P., Kichler A., et al. An imidazole modified lipid confers enhanced mRNA-LNP stability and strong immunization properties in mice and non-human primates. Biomaterials. 2022;286:121570. [PMC] [PubMed]
Veiga N., Diesendruck Y., Peer D. Targeted lipid nanoparticles for RNA therapeutics and immunomodulation in leukocytes. Adv. Drug Deliv. Rev. 2020;159:364–376. [PubMed]
Zhang J., Shrivastava S., Cleveland R.O., Rabbitts T.H. Lipid-mRNA Nanoparticle Designed to Enhance Intracellular Delivery Mediated by Shock Waves. ACS Appl. Mater. Interfaces. 2019;11:10481–10491. [PMC] [PubMed]
Zhang Y., Sun C., Wang C., Jankovic K.E., Dong Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021;121:12181–12277. doi: 10.1021/acs.chemrev.1c00244. [PMC] [PubMed]
---...---
Bio-Synthesis Inc. is pleased to offer a large variety of oligonucleotides (DNA, RNA, siRNA, mRNA, miRNA, etc.) and peptides for a number of research applications, including LNPs, COVID 19 peptides, analysis and vaccine development!
---...---