|
 |
As the capabilities of RNA analysis expand, the need to obtain a standardized RNA fragment has become increasingly important in the development of molecular diagnostic techniques. It can be used as a calibrator, endogenous and exogenous standard for use in QRT-PCR to measure change in levels of a target sequence relative to the same sequence in a known calibrator sample for accurate diagnostic test results1. These RNA can be added and quantified at will, making them very important as reliable controls for each step of the RT-PCR protocol to ensure the accuracies of diagnostic test results. Many RNA based applications not only uses short RNA but very long RNA sequences. The typical RNA control fragment for RT-PCR, Spike-in control or transcript/mRNA for protein synthesis, require RNA fragment between 200 bases and a few thousand bases. RNA synthesis using conventional method can only obtain fragment <100 bases and it is very difficult in obtaining higher yield and quality with much longer sequences. Bio-Synthesis is uniquely positioned and capable to synthesizing long RNA fragment with greater length, quality and fast turnaround time.
Bio-Synthesis provides two types of Long RNA
1. Synthetic Long RNA of >200-250 bases
2. Long RNA transcripts of >3000 bases
|
|
 |
 |
RNA control template for RT-PCR |
 |
Quantified RNA control for real-time RT-PCR |
 |
External RNA spike-in control assay |
 |
In-situ Hybridization RNA probe |
 |
Expression control via anti-sense RNA |
 |
RNA template for in vitro translation2 |
| |
|
|
 |
|
 |
 |
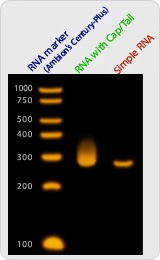
Long RNA fragment: up to few thousand bases |
 |
Quantified RNA fragment |
 |
High yield production: between 20 ug to 1 mg |
 |
Delivered in lyophilized format for long term storage |
 |
High purity of long RNA fragment |
 |
Fast turnaround of 5-10 days |
 |
Reproducible RNA fragment synthesis |
|
|
|
| Reference: |
| 1. |
Kleiboeker, Steven B. "Applications of competitor RNA in Diagnostic Reverse Transcription-PCR." Journal of clinical Microbiology, May 2003, p. 2055–2061 |
|
|
| 2. |
Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, (2nd Ed.), 18.82-18.84. |
|
|
 |
|